When winter arrives and the temperature drops, nature presents us with one of its most enchanting phenomena: snowflakes. These delicate, intricate ice crystals not only transform the world into a winter wonderland but also captivate our imagination with their unique and individual designs. Have you ever wondered how snowflakes form and why each one is distinct? In this blog post, we will explore the mesmerizing process of snowflake formation and delve into the scientific reasons behind snowflake uniqueness.
What Is Needed for Snowflake Formation?
Snowflakes are tiny ice crystals that form high up in the sky, where the air is cold and moist. How they look depends on many factors, such as temperature, humidity, and the weather.
- Temperature is very important for snowflakes to form. So how cold does it have to be to snow? The air has to be colder than freezing (32°F or 0°C) for water vapor to turn into ice. When the air gets cold enough, vapor molecules slow down and stick together, making ice crystals.
- Humidity is how much water vapor is in the air. The more humid the air is, the bigger and more complex snowflakes can get. Water vapor sticks to small things in the air, like dust or pollen, and makes a tiny core for an ice crystal to grow on.
- The weather also affects snowflake formation. When the air is calm and steady, ice crystals can grow in clear and beautiful shapes. When the air is rough or changing quickly, ice crystals grow unevenly and have less detail.
How Snowflakes Are Formed
During their formation, snowflakes undergo the following key stages: nucleation, growth, and branching.
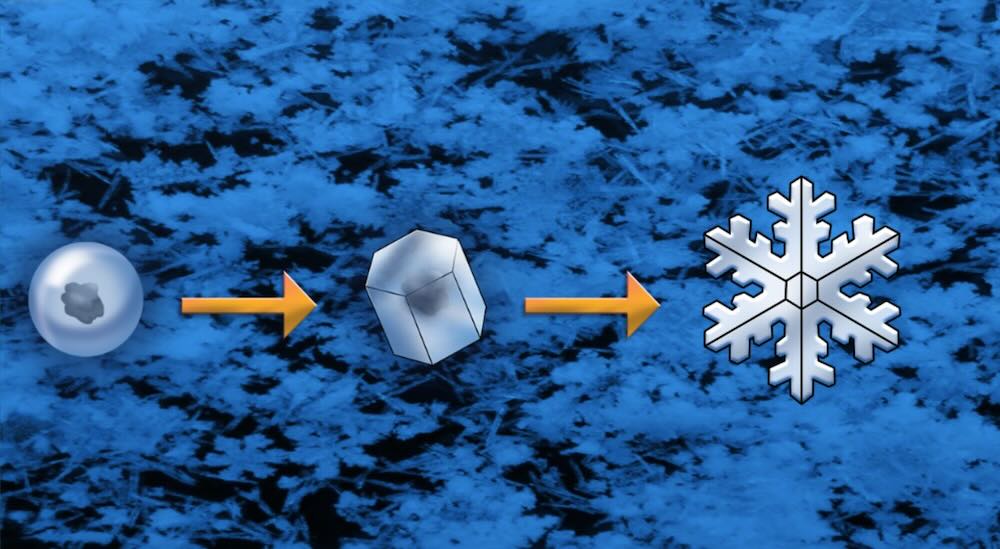 Image source: ABC57
Image source: ABC57
The nucleation process in snowflakes is the first step in ice crystal formation. It occurs when water molecules in the atmosphere come together and organize themselves around tiny particles, such as dust, pollen, or salt. These particles act as nucleation sites, providing a surface for the water molecules to adhere to. Without these particles, it would be more difficult for ice crystals to form spontaneously.
After the water molecules attach to the nucleation site, they begin to arrange themselves in a hexagonal lattice structure. This hexagonal symmetry is a result of the molecular structure of ice. When water freezes, its molecules create the foundation for the six-sided symmetry we associate with snowflakes.
Each snowflake is different because it grows in different ways depending on the weather. The ice crystal gets bigger as more water molecules freeze onto it, making many layers. This process is called accretion, where water molecules in the air freeze onto the existing crystal. The way the crystal grows changes with the temperature and humidity of the air. Sometimes, different parts of the crystal grow faster or slower than others, making branches. The snowflake becomes more and more unique as it grows and falls through different parts of the sky.
Unique Snowflake Patterns
Wilson Bentley, also known as the “Snowflake Man,” was a Vermont farmer who dedicated his life to photographing snowflakes. Between the late 19th and early 20th centuries, Bentley captured over 5,000 images of individual snowflakes under a microscope (micrographs), revealing their intricate and unique structures. Bentley’s work demonstrated the incredible diversity of snowflake shapes. The Jericho Historical Society in Vermont houses the Snowflake Bentley Museum, dedicated to preserving the work and legacy of Wilson Bentley. Visitors can explore Bentley’s original snowflake micrographs and learn about the science of snowflake formation.
 Image source: Wikipedia
Image source: Wikipedia
So why is each snowflake unique? Let’s find out.
Atmospheric Conditions
Although they share a common hexagonal structure, each snowflake undergoes its own journey. As it falls to the ground, it encounters a multitude of environmental factors that shape its growth and design. The huge number of possible combinations and variations ensures that no two snowflakes are ever identical.
Composition of the Atmosphere
The composition of the atmosphere affects snowflake formation as well. Different impurities, such as dust particles or atmospheric pollutants, can influence crystal growth and alter the final shape of the snowflake. This interaction between the crystal and its environment adds another layer of uniqueness to each snowflake’s design.
 Image source: Tim Garrett, the University of Utah, NSF.gov
Image source: Tim Garrett, the University of Utah, NSF.gov
Supercooling
Supercooling is a fascinating weather phenomenon that also plays a significant role in shaping diverse snowflake structures. Supercooling occurs when water remains in a liquid state below its freezing point, typically because it lacks nucleation sites to initiate the solidification process. As a result, water droplets in the atmosphere can exist as supercooled droplets even at temperatures well below freezing.
When an ice crystal meets a supercooled water droplet, it acts as a nucleation site, instantly freezing the droplet upon contact. This rapid freezing process triggers the growth of the ice crystal, which now has a new platform to expand from. The branching arms and intricate details of snowflakes often develop through this process of supercooling. Each encounter with supercooled water allows the crystal to grow uniquely and asymmetrically.
Snowflake Morphology: Types of Ice Crystals
In the early 20th century, Ukichiro Nakaya, a Japanese physicist, conducted extensive research on ice crystals and developed a classification system based on their shapes. He was also the first person in the world who created artificial snow in 1936. His findings are presented in the Nakaya Ukichiro Snow and Ice Museum in Kaga, the scientist’s hometown.
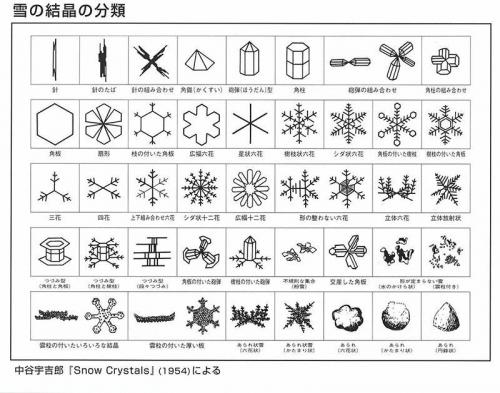 Image source: Kaga General Services, Japan Experience
Image source: Kaga General Services, Japan Experience
We’ve summarized the most common types of ice crystals in a table below.
| Shape | Description |
|---|---|
| Dendrite | Classic snowflake with a six-branched, star-like structure, often large and intricately detailed. |
| Column | Elongated, cylindrical snowflake with hexagonal facets along its length. |
| Plate | Flat, hexagonal snowflake with limited branching, resembling a thin plate. |
| Needle | Long, slender snowflake with a needle-like shape. |
| Columnar Dendrite | A combination of column and dendrite forms, featuring a central column with branch-like arms. |
| Capped Column | Columnar snowflake with flat, disc-like ends. |
| Irregular Aggregates | Clusters of snow crystals that are irregularly shaped and randomly aggregated. |
| Rimed Snowflakes | Snowflakes that have accumulated frozen water droplets (rime) on their surfaces. |
| Graupel | Snowflakes that have gone through a process of riming and become rounded ice pellets. |
Why Do Snowflakes Have Six Sides and Other Interesting Facts
Snowflakes have six sides because of how ice is made. Each water molecule has one oxygen and two hydrogen atoms, which look like a V. When water freezes, these molecules stick together in a way that makes six sides.
Here are some other interesting facts about snowflakes:
- The largest snowflake. According to the Guinness World Records, the largest snowflake ever recorded fell during a snowstorm in Fort Keogh, Montana, USA, on January 28, 1887. This massive snowflake measured about 15 inches (38.1 cm) in diameter.
- Twelve-sided snowflakes. While six-sided snowflakes are the most common, snowflakes with more sides can occur under certain conditions. For example, in December 2022, a photographer from Colorado captured a closeup of a stunning 12-sided snowflake.
 Image source: Jason Persoff
Image source: Jason Persoff
Diamond dust snowflakes. Under certain atmospheric conditions, especially in extremely cold temperatures with very low humidity, a rare phenomenon known as “diamond dust” can occur. It involves the formation of tiny ice crystals in the air, creating the illusion of glittering diamond-like particles floating in the sky.
Snowflakes in space. In 2016, a young star called V883 Orionis had a ring of snow around it. Scientists discovered it by using the ALMA telescopes. Snow in space happens when water, which takes the form of a gas, gets too far from the star and turns into ice. Water gas usually freezes closer to the star than other gases, so it’s hard to see from Earth because the star is too bright. But V883 Orionis had a sudden burst of heat that pushed the snow line farther away – about as far as Pluto is from the Sun. This made it easier for the scientists to see the snow.
 Image source: NRAO • ALMA
Image source: NRAO • ALMA
Conclusion
Snowflakes are a true wonder of nature, mesmerizing us with their beauty. Understanding the process of snowflake formation allows us to appreciate their uniqueness even more. As ice crystals descend from the sky, ever-changing atmospheric conditions influence their growth, resulting in captivating designs. Each snowflake represents a singular journey through the atmosphere, embodying the complexity and diversity of our nature. So, the next time you encounter a snowfall, take a moment to marvel at the intricate masterpieces that are snowflakes.






